Clinovo is a Clinical Research Organization (CRO) that partners with life science companies to streamline their clinical trials. Their CTO Marc Desgrousilliers is managing the development of ClinCapture, their open source Electronic Data Capture (EDC) system. In this interview, he tells us more about why healthcare needs open source and why it is the future of clinical trials.
Read more in this interview with Marc Desgrousilliers.
Marc Desgrousilliers joined Clinovo in 2010, bringing with him over 20 years of experience in software and engineering, and a passion for developing the right system for each customer. His technical IT expertise includes developing patented technologies in storage and network management. Marc has worked with both startups and large international companies throughout the Silicon Valley, including VERITAS, where Marc ran a large worldwide engineering organization, and Microsoft where he led the development of the network management services on Windows.

How does Clinovo leverage open source technologies for a variety of uses?
Clinovo leverages open source technologies for a variety of uses. Our Electronic Data Capture system, ClinCapture, can be run on Linux and uses PostreSQL for its backend. The system can integrate with other eClinical systems, leveraging open source Tomcat and Apache web services. ClinCapture has been interfaced with LimeSurvey to offer the ePRO capability mentioned above, as well as the open source solution Pentaho, to provide real-time graphical reports to data managers and decision-makers.
Clinovo also developed CDISC Express, an open source clinical data conversion tool. CDISC® (Clinical Data Interchange Standards Consortium) established new clinical data standards to speed up data review and improve clinical data exchange, storage, and archival. CDISC Express is a powerful SAS®-based mapping tool that streamlines and automates data conversion process. CDISC Express is an open source solution for advanced clinical SAS programmers to convert clinical data into these recognized standards. With more than 900 downloads, this system is gaining rapid market acceptance.
Why do you believe open source is the future of clinical trials?
Clinical trials are processes that assess the safety and efficacy of a new drug candidate for market application. Clinovo developed ClinCapture, an open source Electronic Data Capture (EDC) system, which collects clinical trial data electronically rather than on paper.
Using an EDC system drastically reduces data entry errors and speeds up the overall clinical data management process, reducing clinical development costs by up to 20%. However, commercial EDC systems come with expensive license fees, pricey professional services as well as inflexible vendor lock-ins.
At Clinovo, we are leveraging open source technologies to answer our sponsors’ need for flexibility and cost-efficiency, without sacrificing on advanced features. A recent study showed that by helping to limit data entry errors and speeding up the clinical trial process, open source EDC systems can bring 47% cost savings compared to paper-based studies.
Electronic Data Capture (EDC) is becoming an increasingly popular solution for streamlining data processing. The essential benefits of EDC are cost-savings, time-savings, patient safety, and compliance with regulatory requirements.
EDC presents the benefit of centralizing data in a single electronic database. It offers a unified, real-time view of the data collected across multiple locations. Cost-savings are substantial thanks to the possibility of remote data monitoring, fewer clinical visits for patients and shorter patient recruitment times, but also reduction (or elimination) of printing costs. Data entry errors are detected earlier than with paper-based systems, lowering the time and cost of data cleaning. Studies show that the duration of clinical development is reduced by up to 30% with EDC systems.
Using EDC also speeds up regulatory reviews by ensuring that the data has been collected, verified and signed in accordance with regulatory requirements. In particular, ClinCapture is 21 CFR Part 11 compliant, ensuring that any data operation is tracked and time-stamped in the system and that data is reviewed and approved by electronic signatures.
How are the end users—the patients being treated for illnesses—ultimately benefiting from these technological advancements?
The substantial time-savings brought about by the use of EDC make drugs available faster to patients, and in some cases saves lives by delivering innovations to the market faster. ClinCapture is integrated with an open source ePRO (Patient Reported Outcomes) system to allow patients to autonomously report their observations from home, thus avoiding time-consuming visits at the clinic. The integration offers automated daily data import from the ePRO system directly into the EDC system. If a patient does not fill the diary as expected, ClinCapture follows up with an automated reminder to the patient the next day.
How does Clinovo exemplify the open source way?
The possibility to easily and freely download the system ensures open exchange. Our roadmap is open and fully accessible; our processes are open and can be reviewed on this wiki. Clinovo is building an online community to constantly enhance the system and better meet the needs of life science companies. We foster the active participation of users and developers in the development of ClinCapture. For example, developers ask questions and offer solutions on our forum to make the installation of ClinCapture more seamless.
The capability of rapid prototyping is part of ClinCapture’s DNA. The latest development version of ClinCapture is released every two weeks, ensuring that the latest innovation is available to the community. Meritocracy is an essential pillar of ClinCapture’s development too. The ticketing system that supports ClinCapture’s development is based on a voting system, giving the opportunity for users and developers to influence the prioritization of new features developments and bug fixes.
Tell us more about ClinCapture.
Historically, ClinCapture focuses on the needs of earlier phase clinical trials with lower budgets and shorter timeframes. However, ClinCapture is well suited to any clinical trial phase or therapeutic area. We have worked on studies from five patients to tens of thousands of patients, showcasing the reliability and stability of the system.
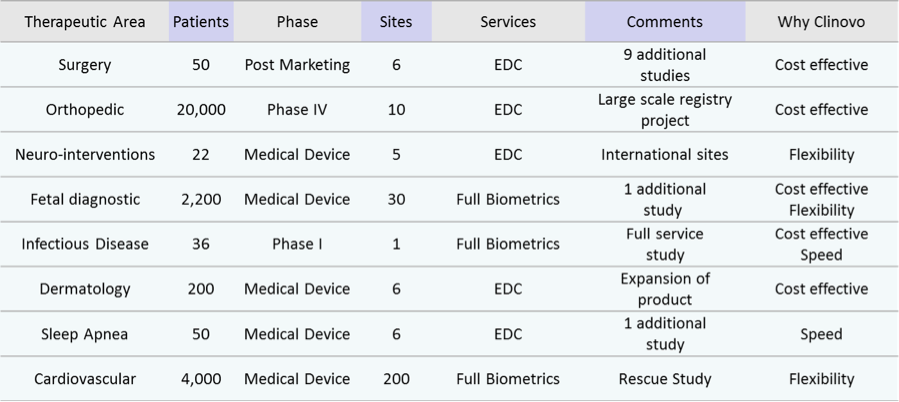
Table 1: Sample of projects performed
We implemented a risk-based validation process and some of the documentation we produce includes: product requirements, specifications, hazard analysis, unit test evidences, code reviews, release notes, build notes, installation protocol and qualification, operation qualification, performance qualification, User Acceptance Testing (UAT), etc. ClinCapture® has been audited multiple times and auditors are pleased with the depth of our validation process and the amount of documentation we provide. Our clients have full access to our documented validation procedures.
Clinovo offers training services for its clients to understand ClinCapture in-depth and get the most out of the system. For example, we trained a global leading medical device company to build new studies themselves. It enabled them to build nine studies so far. In addition, the sponsor was able to host the system in their own datacenter, thus avoiding vendor lock-in and keeping full control over their IT environment.
This approach allowed the client to significantly reduce the overall cost of these post-marketing studies: an estimated 50% of the initial EDC implementation costs were saved. Life sciences companies can benefit from our online community to give their feedback on the system faster and easier than with any other system. We empower them to roll out new studies faster and to be more competitive within their area of expertise.
How does making ClinCapture open source bring you closer to your customers?
We strongly value relationships with our customers and constantly thrive to achieve full customer satisfaction. Clinovo provides innovative solutions to lower the cost of clinical trials while ensuring the same level of quality and sophistication than proprietary systems. We strive to provide quality services, in a timely manner, at a reasonable cost and 80% of our customers choose to do repeat business with us.
Our competitors require expensive license fees, and offer much less flexibility (if any) in the customization of their systems. We commit to match all our clients needs by adapting the system when necessary. Recently, Clinovo interfaced ClinCapture with a randomization system provided by www.randomized.net. To do this, we created a new type of CRF capable of communicating with this IWR system using an encrypted protocol. The IWR system is returning a randomized treatment group for the subject and ClinCapture automatically assigns the subject to a treatment group. This is a premiere in the open source EDC world.
What do you see changing in healthcare technology that is promising and aligns with open source software solutions and the open source way?
The Affordable Health Care Act is pushing for the development of Electronic Medical Records (EMR). This is promising for a greater data integration, which could greatly benefit clinical trials. It will enable clinical trial professionals to select and recruit patients better and understand clinical outcomes more in-depth, given that the patient data is available and can be linked to the data collected during the clinical trial.In clinical trials, cloud technologies are a new opportunity to lower skyrocketing costs.
Electronic Data Capture (EDC) systems, Clinical Trial Management Systems (CTMS), or ePRO systems would be configured and implemented at a much faster pace and at a much lower cost. In January 2012, Forbes calculated the average cost of bringing a new drug to market at $1.3 billion (at times $4B to $11B for big pharmaceutical companies), this calculation takes failed drug applications in account. Thanks to the always-on and automated properties of the cloud, drug development cost is bound to decrease since clinical trials will be started and ended faster than ever before. Read more on the topic at Clinovo’s blog.
Read more about time/cost savings when using open source EDC
in this white paper: The Return on Investment of Open Source Data Integration





3 Comments