Last week, I participated in a panel discussion at the Eastern District of Texas/Federal Circuit Joint Bench-Bar Conference in Dallas. The Court of Appeals for the Federal Circuit is the specialized appellate court for patent cases. My panel was on corporate counsel opinions of patent litigation and recent judicial and legislative patent reform. The discussion was moderated by Judge Richard Linn of the Federal Circuit. It was a great opportunity to present some views of the patent system, and to provide options for improvement to the very people who can enact judicial change.
In my remarks, I pointed out that while there have been some significant judicial changes over the last five years (regarding damages, injunctions, obviousness, indirect infringement, and willfulness), much is still needed. As my colleague Rob Tiller has repeatedly discussed software patents exact considerable costs to innovation in this country. Although we are still waging the war on patent coverage for software, other battles are also in play, which I present here.
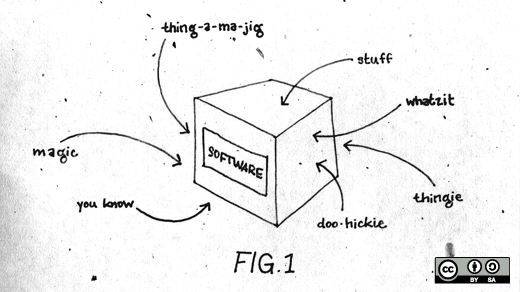
The points I made in Dallas focused on the basic business need for certainty, or at least some degree of predictability, since making efficient and effective business decisions requires a reasonable understanding of the future. The patent system, especially as it relates to software, does not provide this predictability. Software patents are frequently particularly vague in scope, whereas chemical patents will often recite a particular structure which is objectively described. For example, any potential infringer should know exactly what molecule is in play when a patent recites "pyridine-3-carboxylic acid" (niacin or vitamin B3).
Similarly, everyone knows that "a writing device comprising a cylindrical lead, and a tubular wooden body having an end-to-end axial channel, said body contiguously surrounding said lead" is a pencil. Even electrical patents have a reasonable degree of specificity since one can "follow the electrons." However, none of this is true with software patents, which often cover abstract concepts rather than specific physical devices or processes.
This is not merely an academic argument because unclear or unknown boundaries do not provide notice of what such patents cover. To fulfill their notice function, which is one of the basic tenets of our patent system, patent claims must delineate the scope of patent rights with sufficient clarity so that a person skilled in the relevant art can reliably determine whether planned activities would infringe. However, a creative plaintiffs attorney is often the only person who knows whether a product might be accused of infringement. Software patentees are often not required to fully describe their ideas or how to achieve them, and are rewarded with broad “flexible” patents that can metamorphose into different identities throughout litigation. For example, patents on now-obsolete ideas are resurrected to read on technologies developed years later. The claims in litigation often bear little, if any, resemblance to the invention described by the inventor.
So what can we do to solve this uncertainty problem? One answer is to simply apply existing rules. For example, the inventor must fulfill the "written description" requirement by describing the claimed invention in sufficient detail that someone of relevant technical expertise can reasonably conclude that the inventor had possession of the claimed invention. This requirement serves both to disclose the technical knowledge upon which the patent is based and to demonstrate that the patentee was in possession of the claimed invention. Further, the "enablement" requirement mandates that the inventor describe the invention so that that someone of relevant technical expertise can make and use the claimed invention. Yet many, if not most, software patents do little to assist a skilled technician to actually recreate the alleged invention, and many do not disclose enough to prove that the patentee is actually in possession of any invention, but rather reveal an unsupported and impracticable idea.
We should apply these same rules to all patents. Currently, biotechnology and pharmaceutical patents are more closely examined by both the Patent Office and by the courts to make certain that the inventor has an invention and has sufficiently described it. This is a significant part of biotech and pharma patent litigation. However, the written description and enablement requirements have been largely glossed over, if not ignored, in the case of software patents. If a plaintiff is going to twist a patent away from the inventor's original meaning, the patent should provide the public – or at least those who might be accused of infringement via such a creative read – notice of this "new meaning" in some manner. Forcing patentees to describe in detail how to practice the invention would give later-accused parties some predictability so that they can avoid infringement.
Until we have some reasonably certain boundaries on software patents, innovators will continue to face risks that they are unable to fully evaluate.





10 Comments